
Struggling to understand Year 11 Chemistry Module 2: Quantitative Chemistry?
Don’t freak out! We’ll cover the content, how to prepare, use class time and smash through Module 2 on the journey towards your Band 6!
Our comprehensive guide to the second Year 11 Chemistry Module (Quantitative Chemistry) will help you measure up the current content precisely.
Quantitative Chemistry is all about computing exactly what quantity of a chemical species is present, produced or being sent in to react.
The fantastic news is that this topic is the most logical next stage at the construction site of your chemistry mastery: You will always need to know how many molecules you’re spooning in to a reaction, however many grams that ends up weighing.
We are building up, up, up! Just like in Module 1, the stuff you’re learning now is, once again, a skill set that will sit under everything else you do for the rest of the course — so let’s dig into it!
When is quantitative chemistry used?
Overview of Module 2: Quantitative Chemistry
How to Get a Band 6 in Module 2: Quantitative Chemistry
When is quantitative chemistry used?
Here’s NASA doing some quantitative chemistry and working out the precise amount of ozone gas in the atmosphere (which started rushing towards zero in the 80s…).
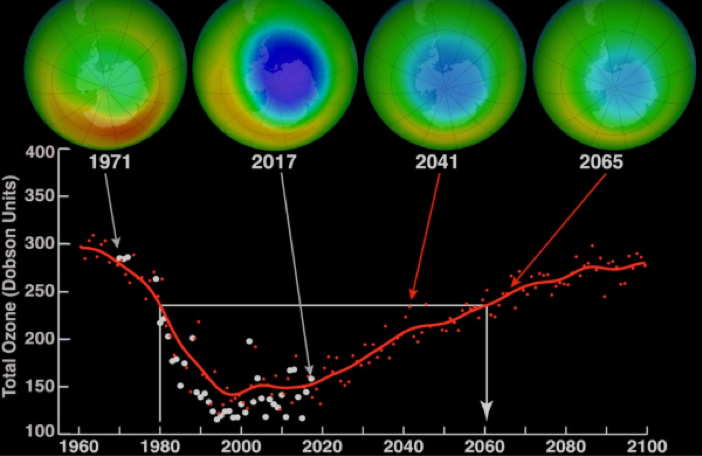
The trend in the 80s (related to the concentration of another gas!), if continued unnoticed would’ve made the Earth’s surface near-uninhabitable. I’m glad someone was counting!
What else?
Picture forensic chemists testifying in court about the exact levels of toxin or DNA found at a crime scene; scientists testing exact chemical levels in food and medicines; environmental and nuclear chemists tracking exact concentrations of radioisotopes, greenhouse gases and pollutants (like lead and mercury).
Picture companies whose reaction products go to market by the tonne for big $$$.
The reactants thrown in at each step along the road to paracetamol, for example, weigh different amounts per molecule. A hundred bowling balls weighs more than 100 golf balls.
The question is “How do you know how much of each to weigh out so they’re in the same number?!”
Picture these things and you’ve got a pretty good idea what quantitative chemistry means for mastering Chemistry overall!
Overview of Module 2: Quantitative Chemistry
So, what exactly is involved with this module?
The problem is: To understand how things really react and behave, we shrink ourselves down and start counting atoms. Which is what we’re doing when we write chemical equations like this:
2Na + Cl2 → 2NaCl
This equation basically says we need 2 sodium atoms for every chlorine molecule.
But up here in the lab, we don’t see atoms! Scales won’t count the atoms in a grain of salt (about a thousand million billion odd); it’d struggle to even tell you the grams.
We must translate masses to particle numbers (in groups of “moles”), do Chemistry, then translate back to masses when talking about products.
Mass is good for knowing how much reactant to weigh out and how much your product will weigh. Chemistry is done in moles.
The first part is all about using reaction ratios to work out masses and volumes.
In the second part you’ll learn how to translate into particle numbers (moles) (“something I can actually work with”) as soon as someone throws you a mass.
In the third part, you’ll be explaining even more that happens in the lab (gas temperature, pressure and volume) in terms of how many moles are there!
Now, let’s break the Quantitative Chem module down!
Inquiry question 1: What happens in chemical reactions?
This is all about calculating and measuring volume and mass changes.
The name of the game is stoichiometry.
If all reactions do is swap around the transient formations taking place in the same bag of atoms, doesn’t the bag always weigh the same?
A party of 200 people has the same total weight, regardless of how they re-arrange their talking circles!
Yes! Reactions are just re-arrangements –
BUT:
If we have reactant vanishing from here, piling in to product over there, we can work some things out!
If the reaction binds atoms in to gas product, we can watch the beaker get lighter as atoms depart for the air!
Inquiry question 2: How are measurements made in Chemistry?
“Human”-scale measurements like mass must be translated to moles so we can actually do Chemistry. You are learning the language of moles!
Inquiry question 3: How does the Ideal Gas Law relate to all other Gas Laws?
If our products or reactants are gaseous under lab conditions, how much space do they take up?
Individual gas molecules generally have so much room to move that they encounter each other rarely, in passing, and their interaction with others doesn’t really determine the behaviour of the gas overall.
In short, their weight, shape/internal structure doesn’t matter – all that matters is… the number of them (how many moles).
Your central question for this module is “How many moles is that?”
Reacting 5 g of Magnesium? …How many moles is that?
The gas product takes up 13.12 Litres… So how many moles is that?
If you’re looking to develop the skills and confidence to master Chemistry, our expert Chemistry tutors near you can provide one-on-one tutoring support in the comfort of your own home or even online.
How to Get a Band 6 in Module 2: Quantitative Chemistry
Here’s what Quantitative Chem begs you to master:
- Reaction ratios:
- Speaking the language of moles fluently
Everything follows from these two.
Step 1: Your new worldview of Chemistry is kinematic.
Recent research shows that the gateway to the concepts in this area (and, chemistry-wide) is seamlessly switching between the lab-world and the micro-world; like being fluent in two languages and saying the same thing in each one.
Once the behaviour of the bulk (viewed from all the way up here: the temperature, pressure, volume, masses that we measure) seems interchangeable and fully reconciled with descriptions of how the many individual particles are behaving; then things are really clicking in place!
If you’re thinking about any chemical reaction at all on the macro-level, STOP and:
Step 1: Visualise what the individual molecules are doing in your head first, then:
Step 2: Do a reaction action-sketch. If a gas product is leaving the reaction site, draw it leaving! Is it coming out hot? What would the bubbling pressure be like?
Here’s an action-shot I’d sketch for Sodium metal reacting with water:
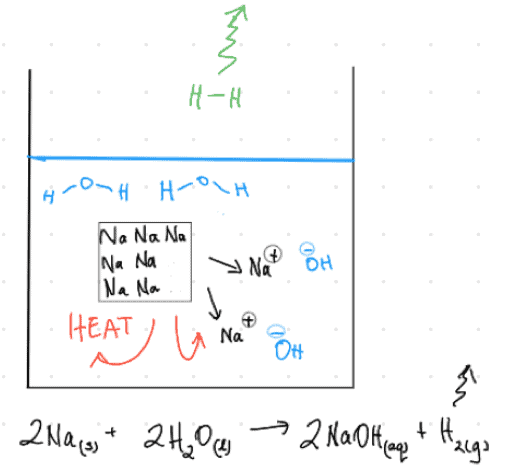
Straight away, I’m thinking of the escaping gases I’ll see (maybe there’s enough heat to vaporise some water too?), the solution’s colour; the fact that if I do this on scales I can watch the mass drop, and so on.
This is a powerful thing to do because it trains your brain to always see the bridge between atom behaviour and visible/touchable/measurable things you see happen when you watch the reaction.
Step 3: Write a short, written explanation of how the situation observed up here (e.g. something is colder, hotter, higher pressure, expanding in volume) arises when averaging over how the trillions of trillions of particles are jostling and jiggling and crashing around.
E.g. How would you explain a wave to a water molecule? Gas pressure to an oxygen molecule
Step 4: Seek out reactions in animated form and get used to visualising them dynamically!
Here’s some great places to find animated chemistry:
PCCL Site
PhET by the University of Colorado
King’s Centre for Visualization in Science
IWant2Study.Org
Step 2: Count everything that happens in moles
In your life (including Chem in junior years), you’ve done everything in mass, down to ordering a “250 g steak” or buying a kilo of Coco Pops or whatever!
So be patient with this transition to moles – students almost always find it mysterious at first.
The problem is that molecules are involved in reactions in exact numbers/ratios, but they all weigh differently – so mass alone tells you nothing about how many reactants and products are going in/coming out.
For example…
Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)
For every 10 H2 molecules out, 10 Mg atoms went in; but they don’t add up to the same weight any more than 10 golf balls and 10 bowling balls add up to the same weight.
Mass is irrelevant, the amounts are important. But how should we count?
A smart bacterium measures its volume in cubic micrometres at about 0.7.
I *suppose* we could measure the volume of something all the way up here with the same units, say “2 million billion cubic micrometres in a milk bottle” …
But… let’s give “1 million billion cubic micrometres” its own name (“litres”) and just say we’ve got 2 of them instead.
“2 litres in a milk bottle.”
The human perspective is on an entirely different scale: A gram of anything is an absurd number of atoms.
Let’s react 2 grams of Hydrogen gas with air.
2H2 + O2 → 2H2O
That’s “about 6 thousand billion million” hydrogen molecules (2 grams), producing “about 6 thousand billion million” water molecules (18 grams! Oxygen’s heavy!).
Let’s… just call exactly that many of them a “mole” and say we have one of those instead.
“1 mole of hydrogen gas reacts, forming 1 mole of water molecules.” Ahh, way better!
You already do this!
For example, “1 ‘dozen’ eggs” for 12. A mole’s just another specific amount with its own name!
Astronomers say, “about 1.2 parsecs”, so they don’t have to say “about 4 thousand million billion centimetres” every time they want to talk about how far away something is (pretty often…).
We’re going to use moles when we want to talk about how many molecules are participating in a chemical reaction (often…).
A mole (6.022×1023) is roughly the number of atoms we’re usually spooning out of containers if we’re weighing out grams.
Specifically, it’s how many atoms make an element weigh as much as its atomic mass, in grams (i.e. 1 gram of H atoms; 12 grams of C atoms, which are 12× heavier).
Don’t worry if you’re still a bit confused!
Keep asking questions, reading and googling things; seek out whatever verification you need to feel totally 100 % happy with what exactly we mean when we talk about “moles”.
Trust me, moles are not going to go away!
Step 3: Practise using the language of moles
Like learning to play guitar or ride a bike. The best way is to do it repeatedly until it start’s happening without thinking.
There are so many mole-practice worksheets on the internet that you Could. Not. Run. Out of practice questions if you tried. It’s not just your school, every other school on the planet has made similar worksheets – Biology and Medical Uni students can end up needing to count in moles as well.
Get your hands on them, consume as many practice questions as you can. Once you don’t have to think about it anymore, it stops getting in the way of the rest of the Chemistry you’re trying to learn (for as long as you keep learning Chemistry!)
Just Google ‘mole practice worksheet’ or take a look in your textbook. Ask your teacher if you’re wanting more!
Run through every mole question in your textbook, and then some.
Step 4: Recast the role of class-time in your life
Because the syllabus is more internationally aligned and focused on pillar topics, there are riches of resources out there already, beyond your prescribed text, that can be used to throw yourself into the content ahead of your class. Some of them will have extras in them, which can be ignored.
Get started by taking a look at Lumenlearning:
Lumenlearning (online course/textbook)
This recasts your class time in the role of a revision session with an expert consultant (teacher), ready to answer your pre-formed questions.
It’s also great to hear concepts explained by different people, using different words, rather than relying on one textbook. Sometimes someone has a brain like yours and explains something just the way you needed to hear it!
In summary, how can I best prepare for the Year 11 Chem Quantitative Chem Module?
Step 1: Always bridge between the atomic world and human worlds when writing chemical equations.
Make sure you can explain observations and measurable things in terms of the activity of the trillions of atoms, as research shows the most successful students do this seamlessly.
Sketch action diagrams of your reactions and reconcile what the atoms are doing with what you would observe happening all the way up here.
Seek out dynamic animations of reactions that you’re studying.
Step 2: Make sure your brain wraps itself around the mole concept.
Otherwise you’re blocking yourself from moving forward!
Once this clicks in you’re like Neo in the Matrix seeing code everywhere and you won’t be able to go back.
Step 3: Practice Mole Questions until it’s automatic!
This is one of those areas where there’s so many practice questions around you could never crunch through them all even if you wanted to.
But put a dent in the world supply of mole practice problems; burn through enough that you start seeing the world in moles instead of grams.
Step 4: Re-cast the role of class time in your life
Don’t make class time your primary learning ground anymore. Make it a revision session that you bring questions to; your teacher becomes your consultant/mentor brought in to help you master this two-year project of yours!
Want to make sure you’re organised for your HSC Chemistry assessments? Start using our HSC Study Notes Notion Template, which includes syllabus dot points to follow when making your study notes! P.S. It’s also got an assessment planner, habit tracker, past papers, and more!
On the hunt for other Year 11 Chemistry resources?
Check out our module guides below:
- Module 1: Structure and Properties of Matter
- Module 3: Reactive Chemistry
- Module 4: Drivers of Reactions
We’ve also got other practice questions which you can try below:
- Properties and Structure of Matter Practice Questions
- Reactive Chemistry Practice Questions
- Drivers of Reactions Practice Questions
Are you looking for some extra help with Year 11 Chemistry Module 2: Quantitative Chemistry?
We pride ourselves on our inspirational Year 11 Chemistry coaches and mentors!
We offer tutoring and mentoring for Years K-12 in a large variety of subjects, with personalised lessons conducted one-on-one in your home or at one of our state of the art campuses in Hornsby or the Hills! We can also provide tutoring support in Western Sydney.
To find out more and get started with an inspirational tutor and mentor get in touch today!
Give us a ring on 1300 267 888, email us at [email protected] or check us out on TikTok!
Adrian Wendeborn is a qualified Science and Maths teacher with a Physics/Chemistry double-major degree from USYD and a GDipEd from UQ. Adrian has taught in QLD and NSW and has worked with Art of Smart Education as a campus teacher, tutor, resource developer and Head of Faculty.


